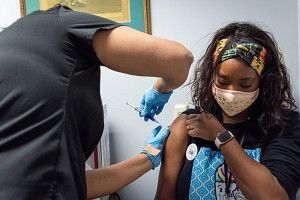
Côte d’Ivoire’s administration of the R21/Matrix-M malaria vaccine on June 15, 2024, marks the first use of this vaccine in efforts to combat malaria among African children.
Image credits: Novavax

On June 15, 2024, Côte d’Ivoire made history by becoming the first country to administer the R21/Matrix-M malaria vaccine to children, a groundbreaking development in the battle against a disease that claimed over 600,000 lives last year, mostly among children under five.
The R21-Matrix-M vaccine, developed by the University of Oxford and the Serum Institute of India, leverages Novavax’s Matrix-M™ adjuvant to enhance immune response. Prequalified by the WHO, it boasts an efficacy rate of 78%, exceeding the WHO’s 75% target, and aims to address the urgent need for malaria vaccines in endemic regions.
The rollout will begin in Abidjan and expand to 38 districts, with 15 African countries expected to introduce malaria vaccines by 2024, targeting 6.6 million children. The Serum Institute of India has manufactured 25 million doses, with plans to produce 100 million annually at under $4 per dose.
Key Takeaways
- Côte d’Ivoire made history as the first country to administer the R21/Matrix-M malaria vaccine on June 15, 2024, targeting malaria’s impact on children.
- The vaccine boasts an efficacy rate of 78% and has received WHO prequalification, making it a significant advancement in malaria prevention.
- Plans are in place to vaccinate 6.6 million children across 15 African countries by the end of 2024, supported by affordable production from the Serum Institute of India.
On May 20, 2024, Novavax partnered with the Serum Institute of India (SII) to dispatch the R21/Matrix-M malaria vaccine. This marked a pivotal step in the process of getting the vaccine administered in regions where the disease is most prevalent.2
“The R21/Matrix-M vaccine is a vital new tool to help stop the devastating health and economic impact of malaria on nearly half of the world’s population, including the tragic loss of 1,300 children every single day,” said Novavax CEO John C Jacobs. “Now more than ever, collaborations are imperative to address unmet needs in preventable infectious disease. Novavax is proud of our partnership with the University of Oxford and SII, and the role of Matrix-M adjuvant in this vaccine, and grateful for the support of Gavi and UNICEF in its rollout.”1
The endorsement of the R21/Matrix-M malaria vaccine by WHO signifies a major step forward in the fight against malaria. If implemented effectively, this vaccine could substantially reduce the burden of malaria-related morbidity and mortality worldwide.
In February 2024 The Lancet published the phase 3 trial of the R21/Matrix-M vaccine demonstrated unprecedented safety, efficacy, and cost-effectiveness. This development is particularly significant given that malaria claims approximately 600,000 lives each year, predominantly among African children.
“In this phase III licensure trial of the R21/Matrix-M malaria vaccine, the primary analysis shows vaccine efficacy against clinical malaria of 75% at seasonal sites and 67% at standard sites across the entire cohort aged 5–36 months over 12 months,” according to The Lancet investigators. “This finding suggests that the R21/Matrix-M vaccine might not only substantially decrease the number of clinical malaria cases, but could also contribute to programs to reduce malaria transmission when used with other interventions, particularly if deployed across a wider age range.”
Throughout this journey, challenges such as vaccine distribution, access, and acceptance in affected regions have been accounted for as significant hurdles. Amidst the jubilation lies the acknowledgment of the challenges ahead. Regulatory hurdles, distribution complexities, and market acceptance pose formidable obstacles, underscoring the arduous journey toward widespread adoption.
Novavax remains committed to transparency, urging stakeholders to scrutinize its filings with the Securities and Exchange Commission (SEC) for a comprehensive understanding of the risks involved. Despite the challenges, Novavax, its partners, and global health organizations are resolute in their determination to combat malaria and protect vulnerable populations.
The rollout of the R21/Matrix-M malaria vaccine in Côte d’Ivoire signifies a significant development in vaccination efforts, offering hope to millions at risk of malaria. As the first country to implement this vaccine, Côte d’Ivoire sets an example for others. With support from global health organizations and efforts to address distribution challenges, this vaccine has the potential to reduce the impact of malaria. This initiative aims to create a future where malaria is no longer a threat to vulnerable populations, ensuring improved health outcomes for children globally.
Reference
- University of Oxford. Côte d’Ivoire makes history as first nation to deploy R21/Matrix-M™ Malaria Vaccine. Medsci.ox.ac.uk. Published July 15, 2024. Accessed July 18, 2024. https://www.medsci.ox.ac.uk/news/cote-divoire-makes-history-as-first-nation-to-deploy-r21-matrix-mtm-malaria-vaccine
- Novavax. First Doses of R21/Matrix-M Malaria Vaccine Shipped to Africa. Published May 20, 2024. Accessed July 18, 2024. https://ir.novavax.com/press-releases/First-Doses-of-R21-Matrix-M-TM-Malaria-Vaccine-Shipped-to-Africa
- Abene, S. The R21/Matrix-M Vaccine: A Breakthrough in Malaria Eradication Efforts. Contagion. Published April 25, 2024. Accessed July 18, 2024. https://www.contagionlive.com/view/the-r21-matrix-m-vaccine-a-breakthrough-in-malaria-eradication-efforts