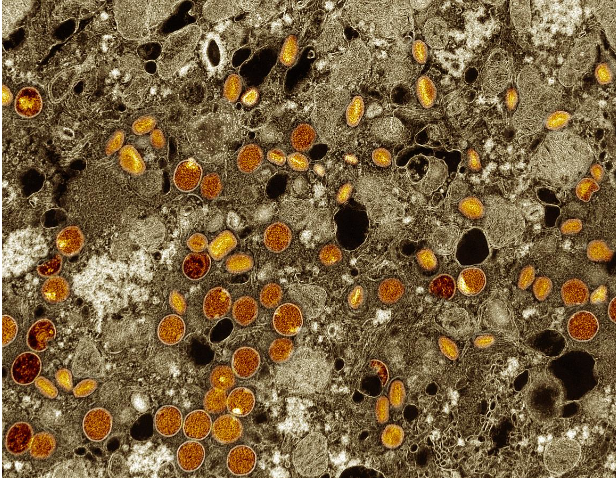
Colorized transmission electron micrograph of mpox virus particles (red/yellow) found within infected VERO E6 cells (brown). The virus particles are in various stages of maturity, which accounts for differences in shape.
Image credits: The NIAID Integrated Research Facility in Fort Detrick, Maryland. NIAID

SIGA Technologies Inc has announced preliminary results from the PALM 007 clinical trial, which assessed the antiviral tecovirimat, also known as TPOXX, for treating monkeypox (mpox) in the Democratic Republic of the Congo (DRC). The trial did not achieve its primary goal of showing a statistically significant improvement in lesion resolution compared with placebo. Although, tecovirimat’s safety profile remained consistent with that of the placebo and aligned with findings from previous studies.1
Despite not meeting the primary endpoint, the study reported a 1.7% mortality rate among participants, significantly lower than the 3.6% mortality rate observed in broader DRC cases. This suggests that high-quality supportive care can improve outcomes.2 Additionally, results indicate that tecovirimat may offer clinical benefits to two key patient groups: Those who started treatment early and those with severe disease, defined as having 100 or more skin lesions.1
“These findings are disappointing, but they give us essential information and reinforce the need to identify other therapeutic candidates for mpox while we continue research on tecovirimat use in other populations with mpox,” said NIAID Director Jeanne Marrazzo, MD, MPH. “We remain committed to developing safe and effective interventions, including treatments and vaccines, that can ease the devastating mpox burden in Central Africa and address the milder form of the virus that is circulating globally.”2
Main Takeaways
- Tecovirimat showed no significant improvement in lesion resolution compared to placebo in the PALM 007 trial, though its safety profile remained consistent with prior studies.
- The study suggested tecovirimat may benefit early-treated patients and those with severe disease, with a lower mortality rate among participants indicating the importance of high-quality supportive care.
- Ongoing trials are investigating tecovirimat’s efficacy further, while international health organizations and the US government are actively responding to the mpox outbreak with emergency measures and support.
READ MORE>> Africa CDC Director General Declares Mpox Outbreak a Public Health Emergency of Continental Security
The PALM 007 trial, part of a global effort addressing the 2022 mpox outbreak, enrolled 597 participants who received either tecovirimat or placebo and were hospitalized for at least 14 days. Results may differ from real-world scenarios due to the controlled environment of the trial, which is why more trials are still ongoing.2
Currently, suspected cases of mpox have traveled across Africa, surging past 17,000, a significant increase from 7146 cases in 2022 and 14,957 cases in 2023. These countries have confirmed 2863 cases and 517 deaths, primarily in the DRC.3 A recent report from the CDC stated, 67% of suspected mpox cases and 87% of suspected mpox deaths in the DRC have involved individuals aged 15 and younger.4
In response to this 2024 outbreak, The Africa Centers for Disease Control and Prevention (Africa CDC) has issued its first-ever Public Health Emergency of Continental Security (PHECS)3 and the World Health Organization has declared the mpox outbreaks in Congo and other African countries a global emergency.5
In response, the US Department of Health and Human Services (HHS) is collaborating with the Africa CDC and WHO to address the outbreak and has provided more than $17 million in recent months for surveillance, community engagement, and vaccination efforts. The US is providing 50,000 doses of the FDA-approved JYNNEOS vaccine (a live, non-replicating product of Bavarian Nordic) to the DRC and collaborating on vaccine distribution. Although there are no current cases in the United States, surveillance systems are in place and high-risk individuals are advised to get vaccinated. The CDC has also issued travel and health advisories to manage potential risks from clade I mpox.6
“The PALM007 study demonstrated the importance and value of testing investigational mpox treatments through robust clinical trials in the DRC’s endemic setting,” said Lori Dodd, PhD, NIAID’s PALM project lead for the DRC. “We’ll continue to evaluate the trial data to determine whether additional studies of tecovirimat in patient subgroups are warranted.2
Further research is ongoing with trials such as STOMP, UNITY, Platinum-CAN, and EPOXI to assess tecovirimat’s efficacy in treating mpox under various conditions. SIGA Technologies and NIAID are analyzing these studies to better understand tecovirimat’s potential benefits. Together, these trials aim to deepen knowledge of tecovirimat and refine its application for managing mpox.1
References
-
SIGA. Topline Results from PALM 007 Study of SIGA’s Tecovirimat in Treatment of Mpox Released. GlobalNewswire. Published August 15, 2024. Accessed August 15, 2024. https://www.globenewswire.com/news-release/2024/08/15/2930825/9738/en/Topline-Results-from-PALM-007-Study-of-SIGA-s-Tecovirimat-in-Treatment-of-Mpox-Released.html
-
NIH. The antiviral tecovirimat is safe but did not improve clade I mpox resolution in Democratic Republic of the Congo. Published August 15, 2024. Accessed August 15, 2024. https://www.nih.gov/news-events/news-releases/antiviral-tecovirimat-safe-did-not-improve-clade-i-mpox-resolution-democratic-republic-congo
-
3. Africa CDC Declares Mpox A Public Health Emergency of Continental Security, Mobilizing Resources Across the Continent. Africa CDC. August 13, 2024. August 15, 2024. https://africacdc.org/news-item/africa-cdc-declares-mpox-a-public-health-emergency-of-continental-security-mobilizing-resources-across-the-continent
-
U.S. Preparedness and Response to Increasing Clade I Mpox Cases in the Democratic Republic of the Congo — United States, 2024. MMWR. Published May 16, 2024. Accessed August 15, 2024. https://www.cdc.gov/mmwr/volumes/73/wr/mm7319a3.htm
-
Cheng M. WHO declares mpox outbreaks in Africa a global health emergency as a new form of the virus spreads. AP News. August 14, 2024. Accessed August 15, 2024. https://apnews.com/article/who-mpox-africa-health-emergency-cc9bdf31b49d06bec5efd44fb55d5e42
-
United States Government’s Response to the Clade I Mpox Outbreak in the Democratic Republic of the Congo and Other Countries in the Region. US Department of Health and Human Services. Published August 14, 2024. Accessed August 15, 2024. https://www.hhs.gov/about/news/2024/08/14/united-states-governments-response-clade-i-mpox-outbreak-democratic-republic-congo-other-countries-region.html