Final Diagnosis
Invasive Fusarium sinusitis
History of present illness
A 74-year-old White man who initially presented at a local urgent care with a 2-day history of progressively worsening pounding frontal headache, generalized weakness, frontal/maxillary sinus tenderness, rhinorrhea, and bilateral blurring of vision. He was diagnosed with presumptive bacterial sinusitis and was sent home on amoxicillin/clavulanate 500 mg every 8 hours for 7 days. Despite being on antibiotics for 2 days, his symptoms progressively worsened, particularly his headache. He eventually developed subjective fevers and chills, prompting him to come to the emergency department.
Medical history
He had a history of high-risk myelodysplastic syndrome (MDS) on decitabine/cedazuridine. He was diagnosed with MDS 2 years ago, and his last cycle of decitabine/cedazuridine was approximately 1 month prior to his presentation. He also had a history of type 2 diabetes mellitus.
Key medications
• Decitabine 35 mg/cedazuridine 100 mg orally once a month
• Metformin 500 mg orally daily
Epidemiological history
He lived with his wife, daughter, and 5 grandchildren. He had 2 pet cats and no contact with any farm animals. He denied gardening, hiking, camping, fishing, and hunting. He had no known sick contacts and no recent travel. He was in the military approximately 50 years ago. He had never been incarcerated, and he denied illicit drug use, cigarette smoking, and alcohol intake. There was no active or recent renovation in his house.
Physical examination
The patient appeared older than his age, not in cardiorespiratory distress but visibly in pain. His vital signs were a blood pressure of 112/71 mm Hg, heart rate of 84 beats per minute, temperature of 98.1 °F (36.72 °C), and respirations of 18 breaths per minute. On physical examination, he had bilateral frontal and maxillary sinus tenderness, warmth, and erythema that was worse on the right. There was also mild but noticeable right-sided periorbital edema. His pupils were equal and reactive to light and accommodation with no pain in movement. The remainder of the physical examination findings were normal.
Studies
His white blood cell count was 0.5 K/μL (reference range, 4.40-11.30 K/μL), with neutrophils at 5%, no bands, lymphocytes at 75%, and monocytes at 10%. The absolute neutrophil count was 0. The hemoglobin was 7.7 g/dL (reference range, 13.8-17.4 g/dL) and hematocrit was 22.9% (reference range, 41%-51%). The platelet count was 39 K/uL (reference range, 150-450 K/μL). His creatinine was 0.88 mg/dL (reference range, 0.6-1.3 mg/dL), blood urea nitrogen level was 21 mg/dL (reference range, 7-20 mg/dL), and glucose was 105 mg/dL. His C-reactive protein level was 136 mg/L (reference range, < 5 mg/L), procalcitonin was less than 0.05, and ferritin was 3456.7 ng/mL (reference range, 26-388 ng/ml). Blood cultures did not grow anything.
Image credit: Ian Adrian F. Frani, MD, BSN

A CT scan (Figure 1) showed mucosal thickening in frontal sinuses, ethmoid air cells, and right maxillary sinus. No acute territorial infarct, intracranial hemorrhage, cerebral edema, or cerebral mass effect is seen. An MRI of the orbits (Figure 2) showed severe paranasal sinus disease involving frontal sinuses, ethmoid air cells, sphenoid sinuses, and right greater than left maxillary sinus. There is no evidence of intracranial or intraorbital extension or other complication. Orbits and brain are otherwise unremarkable.
Clinical course
Infectious diseases service was consulted, and because of his febrile neutropenia and concern for invasive sinusitis, he was started on intravenous liposomal amphotericin 4 mg/kg/day, vancomycin, and piperacillin/tazobactam to cover for fungal and bacterial sinusitis, respectively. Otorhinolaryngology evaluated the patient and took him to surgery for endoscopic sinus surgery with right maxillary antrostomy and right ethmoidectomy. A frozen section was done intraoperatively and noted fungal elements, with no obvious vascular invasion.
Image credit: Ian Adrian F. Frani, MD, BSN

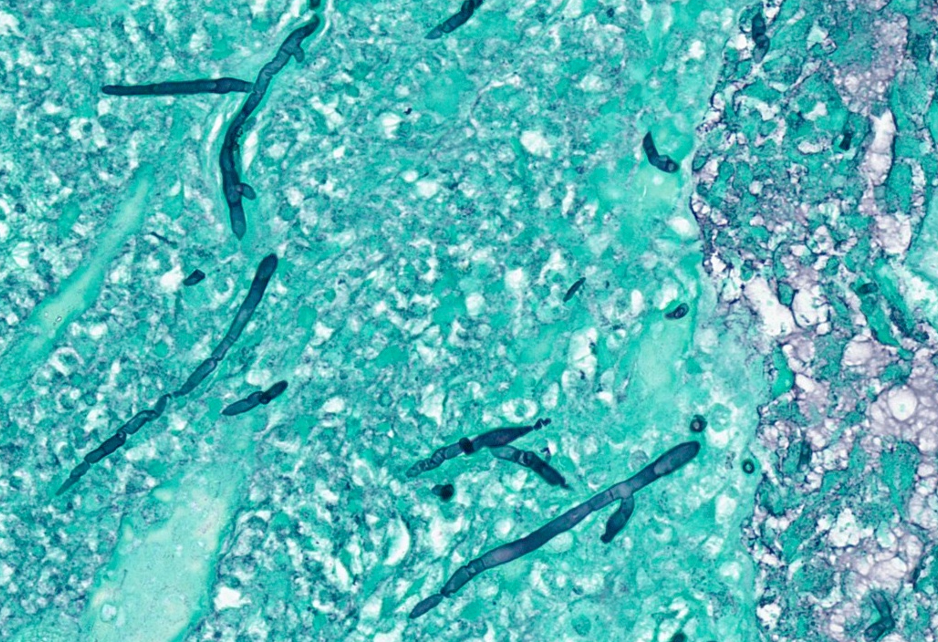
Pathology of tissue samples showed necrotic tissue with numerous fungal organisms with long, thin hyphal forms with regular septations and acute angle branching (Figure 3). The tissue samples that were sent for culture grew mold and were eventually identified as Fusarium species. Empiric vancomycin and piperacillin/tazobactam were stopped, and voriconazole was added in addition to liposomal amphotericin B for 1 week post procedure and was eventually transitioned to voriconazole 200 mg orally twice-a-day monotherapy. Granulocyte colony-stimulating factor (G-CSF) was administered with consultation from oncology to shorten the period of neutropenia. Because of concerns for recurrence and the high risk of mortality associated with invasive fungal disease in this population, a decision was made to keep him on voriconazole long term with serum level monitoring for at least 3 to 6 months, or at least until immunosuppression and neutropenia have resolved whichever is the latest. His symptoms resolved a few days after debridement, and he was eventually discharged. Two weeks later, he was readmitted for a fever and worsening headache and periorbital pain. Repeat MRI showed worsening sinusitis without any intracranial and intraorbital extension. This time, his blood cultures showed Clostridium septicum. He was started on piperacillin/tazobactam and liposomal amphotericin B in addition to voriconazole. He was then transferred to a higher-level facility where he underwent endoscopic debridement twice prior to being discharged on voriconazole in addition to levofloxacin and acyclovir prophylaxis. Unfortunately, he was readmitted after 1 month with recurrent worsening sinusitis, and after a few weeks, his family opted for palliative and comfort care.
Image credit: Ian Adrian F. Frani, MD, BSN

Discussion
Fusarium species are widely distributed as water structure biofilms in soil and water and are important plant pathogens.1 They can cause hyalohyphomycosis similar to Aspergillus, Penicillium, and Scedosporium species, among others.2 Hyalohyphomycosis is a term used to described opportunistic fungal infections caused by molds whose basic tissue form is in the nature of hyaline, light-colored, hyphal elements that are branched or unbranched, occasionally toruloid, and without pigment in their wall.2,3 Microscopically, they resemble Aspergillus species in that they have septated hyaline hyphae 3 to 8 μm in diameter that branch at acute or right angles and produce both fusoid macroconidia and microconidia, which is a characteristic of the genus Fusarium.2 There are more than 50 species of Fusarium, but only 12 species cause human disease.1 Fusarium solani complex is the most pathogenic of the species4 and is most frequently associated with human disease (approximately 40%-60% of cases), followed by Fusarium oxysporum (20%), Fusarium verticillioides (10%), and Fusarium moniliforme (10%).1,5
Fusarium species can produce trichothecenes and other mycotoxins that can suppress humoral and cellular immunity, as well as cause tissue breakdown. Because of its virulence factors and interplay with the host’s immune system, particularly innate immunity and T-cell defenses, the clinical manifestation and its severity are largely dictated by the degree of immune suppression.1,4 Immunocompetent hosts usually have more localized presentation in contrast to more invasive and disseminated disease associated with immunocompromised hosts. Prolonged neutropenia and recent corticosteroid therapy are associated with poor outcomes in the immunocompromised population.1,2,4 Mortality in the immunocompromised patients has been reported to range from 50% to 80%.1,2
In a retrospective study by Nucci et al that included 84 patients with hematologic cancers and invasive fusariosis, factors associated with poor survival included persistent neutropenia and corticosteroid therapy. The actuarial survival rates in the study are 0% in patients with both prolonged neutropenia and recent corticosteroid therapy, 4% for those with only prolonged neutropenia, and 30% for those on recent corticosteroids.6 In this case, our patient is not on corticosteroids but is on decitabine/cedazuridine, which can cause myelosuppression and increase risk of infection.
Diagnosis is made by identification of the fungus via growth from a culture such as blood, skin, corneal scrapings, and lung/nasal tissues.1,4 Fusarium grows readily on blood cultures but can grow 10 to 35 hours faster, depending on the subspecies, if grown on fungus-specific media.1 This is in contrast with Aspergillus, which rarely grows on blood cultures (5% recovery rate in Aspergillus vs 50% in Fusarium).7 This characteristic of Fusarium to grow on blood cultures can be attributed to adventitious sporulation, which is the production of reproductive fungal structures in infected human tissue, including blood.7 Identification is important because Aspergillus and Fusarium can resemble each other morphologically and can both invade blood vessels, causing thrombosis, tissue infarction, and eventually necrosis.2
Management of fusariosis includes a combination of antifungal therapy, decreasing the immunosuppression, and, in cases of localized infection, surgical debridement. Fusarium species are intrinsically resistant to echinocandins.7 It can also develop resistance mechanisms such as changes in amino acid sequences and overexpression of genes that can both promote azole resistance and efflux pumps.7 Voriconazole and amphotericin B are the drugs of choice, with posaconazole being used for salvage therapy.4,5 Because Fusarium is highly resistant and in vitro susceptibility to various antifungals are low and unreliable with no established minimal inhibitory concentrations, combination therapy is recommended, especially if susceptibility has not returned in immunocompromised patients with invasive or disseminated disease.2,4,5 There are no defined guidelines for duration of therapy, but because of the high risk of relapse in immunocompromised patients, prolonged therapy with secondary prophylaxis is recommended.4 Administration of G-CSF should be considered to shorten the duration of neutropenia, although its benefit on mortality in this population has not been established.1,2,4
Fusariosis has a wide variety of clinical manifestations that are directly related to the degree of immunosuppression.
As our patient highlights, there should be a high degree of suspicion for invasive or disseminated fusariosis in patients who are severely ill with prolonged neutropenia and significant immunosuppression because mortality is high, even on appropriate antifungal therapy, in this population.
References
-
Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007;20(4):695-704. doi:10.1128/CMR.00014-07
-
Dignani MC, Anaissie E. Human fusariosis. Clin Microbiol Infect. 2004;10(suppl 1):67-75. doi:10.1111/j.1470-9465.2004.00845.x
-
Ajello L. Hyalohyphomycosis and phaeohyphomycosis: two global disease entities of public health importance. Eur J Epidemiol. 1986;2(4):243-251. doi:10.1007/BF00419488
-
Tortorano AM, Prigitano A, Esposto MC, et al. European Confederation of Medical Mycology (ECMM) epidemiological survey on invasive infections due to Fusarium species in Europe. Eur J Clin Microbiol Infect Dis. 2014;33(9):1623-1630. doi:10.1007/s10096-014-211-1
-
Batista BG, Chaves MA, Reginatto P, Saraiva OJ, Fuentefria AM. Human fusariosis: an emerging infection that is difficult to treat. Rev Soc Bras Med Trop. 2020;53:e20200013. doi:10.1590/0037-8682-0013-2020
-
Nucci M, Anaissie EJ, Queiroz-Telles F, et al. Outcome predictors of 84 patients with hematologic malignancies and fusarium infection. Cancer. 2003;98(2):315-319. doi:10.1002/cncr.11510
-
Liu K, Howell DN, Perfect JR, Schell WA. Morphologic criteria for the preliminary identification of Fusarium, Paecilomyces, and Acremonium species by histopathology. Am J Clin Pathol. 1998;109(1):45-54. doi:10.1093/ajcp/109.1.45